上海金畔生物科技有限公司为生命科学和医药研发人员提供生物活性分子抑制剂、激动剂、特异性抑制剂、化合物库、重组蛋白,专注于信号通路和疾病研究领域。
Fevipiprant (Synonyms: QAW039; NVP-QAW039) 纯度: 99.63%
Fevipiprant (QAW039; NVP-QAW039) 是一种有选择性的,强有力的,可逆的竞争性拮抗剂。靶点为CRTh2受体,KD值为1.1 nM,IC50为0.44 nM,能够引发人体嗜酸性粒细胞的形状变化。
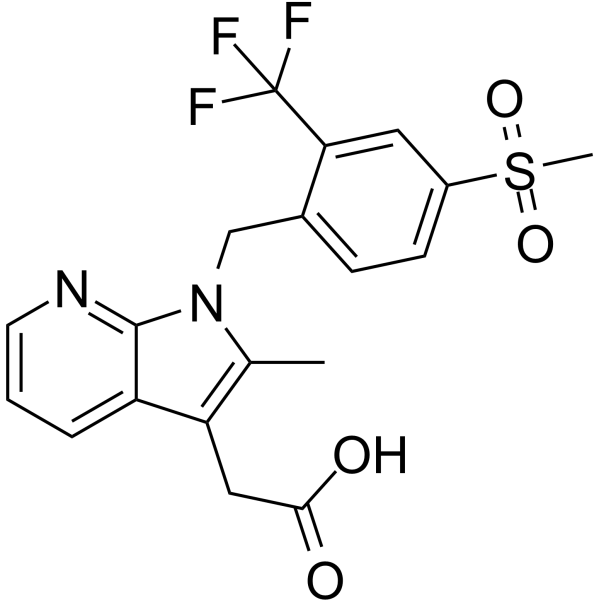
Fevipiprant Chemical Structure
CAS No. : 872365-14-5
| 规格 | 价格 | 是否有货 | 数量 |
|---|---|---|---|
| Free Sample (0.1-0.5 mg) | Apply now | ||
| 10 mM * 1 mL in DMSO | ¥825 | In-stock | |
| 5 mg | ¥750 | In-stock | |
| 10 mg | ¥1100 | In-stock | |
| 25 mg | ¥2200 | In-stock | |
| 50 mg | ¥3300 | In-stock | |
| 100 mg | ¥5500 | In-stock | |
| 200 mg | ¥8800 | In-stock | |
| 500 mg | 询价 | ||
| 1 g | 询价 |
* Please select Quantity before adding items.
Fevipiprant 相关产品
•相关化合物库:
- Drug Repurposing Compound Library Plus
- Clinical Compound Library Plus
- Bioactive Compound Library Plus
- GPCR/G Protein Compound Library
- Immunology/Inflammation Compound Library
- Anti-Cancer Compound Library
- Clinical Compound Library
- Drug Repurposing Compound Library
- Endocrinology Compound Library
- Anti-COVID-19 Compound Library
- Orally Active Compound Library
- Angiogenesis Related Compound Library
- Children’s Drug Library
| 生物活性 |
Fevipiprant (QAW039; NVP-QAW039) is a selective, potent, reversible competitive CRTh2 antagonist with an in vitro dissociation constant KD value of 1.1nM at the CRTh2 receptor and an IC50 value of 0.44 nM for inhibition of PGD2-induced eosinophil shape change in human whole blood. IC50:0.44 nM(PGD2-induced eosinophil shape change) Kd value:1.1nM(CRTh2 receptor)[1] In vitro: CRTh2-mediated shape change in eosinophils was used to profile QAW039 in whole blood and represents a physiologically relevant environment. The comparable IC50 values for QAW039 in the whole blood and isolated shape-change assays are consistent with its lower plasma-protein binding and its relatively slow dissociation kinetics that drive its increased potency .QAW039 is highly potent in whole-blood systems, with the IC50 value obtained consistent with the affinity values calculated from radioligand experiments. In a further disease-relevant cellular context, the potency of QAW039 in the isolated Th2 cell cytokine inhibition assay is consistent with its CRTh2 receptor affinity, and, as with eosinophil assay readouts, this represents an improved potency compared with QAV680[2]. |
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Clinical Trial |
|
||||||||||||||||
| 分子量 |
426.41 |
||||||||||||||||
| Formula |
C19H17F3N2O4S |
||||||||||||||||
| CAS 号 |
872365-14-5 |
||||||||||||||||
| 运输条件 |
Room temperature in continental US; may vary elsewhere. |
||||||||||||||||
| 储存方式 |
|
||||||||||||||||
| 溶解性数据 |
In Vitro:
DMSO : ≥ 32 mg/mL (75.05 mM) * “≥” means soluble, but saturation unknown. 配制储备液
*
请根据产品在不同溶剂中的溶解度选择合适的溶剂配制储备液;一旦配成溶液,请分装保存,避免反复冻融造成的产品失效。 In Vivo:
请根据您的实验动物和给药方式选择适当的溶解方案。以下溶解方案都请先按照 In Vitro 方式配制澄清的储备液,再依次添加助溶剂: ——为保证实验结果的可靠性,澄清的储备液可以根据储存条件,适当保存;体内实验的工作液,建议您现用现配,当天使用; 以下溶剂前显示的百
|
||||||||||||||||
| 参考文献 |
|
所有产品仅用作科学研究或药证申报,我们不为任何个人用途提供产品和服务
