上海金畔生物科技有限公司为生命科学和医药研发人员提供生物活性分子抑制剂、激动剂、特异性抑制剂、化合物库、重组蛋白,专注于信号通路和疾病研究领域。
Ruxolitinib sulfate (Synonyms: INCB018424 sulfate)
Ruxolitinib sulfate (INCB018424 sulfate) 是一种有效的 JAK1/2 抑制剂,IC50 值为 3.3 nM/2.8 nM,选择性是对 JAK3 的 130 倍。
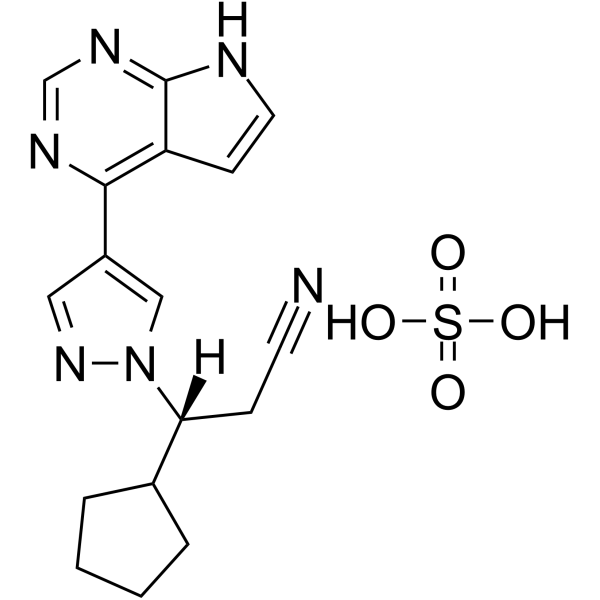
Ruxolitinib sulfate Chemical Structure
CAS No. : 1092939-16-6
| 规格 | 是否有货 | ||
|---|---|---|---|
| 100 mg | 询价 | ||
| 250 mg | 询价 | ||
| 500 mg | 询价 |
* Please select Quantity before adding items.
Ruxolitinib sulfate 的其他形式现货产品:
| 生物活性 |
Ruxolitinib sulfate (INCB018424 sulfate) is the first potent, selective JAK1/2 inhibitor to enter the clinic with IC50s of 3.3 nM/2.8 nM, and has > 130-fold selectivity for JAK1/2 versus JAK3. |
||||
|---|---|---|---|---|---|
| IC50 & Target[1] |
|
||||
| 体外研究 (In Vitro) |
Ruxolitinib sulfate (INCB018424 sulfate) potently and selectively inhibits JAK2V617F-mediated signaling and proliferation, markedly increases apoptosis in a dose dependent manner, and at 64 nM results in a doubling of cells with depolarized mitochondria in Ba/F3 cells. Ruxolitinib demonstrates remarkable potency against erythroid colony formation with IC50 of 67 nM, and inhibits proliferating of erythroid progenitors from normal donors and polycythemia vera patients with IC50 values of 407 nM and 223 nM, respectively[1]. 上海金畔生物科技有限公司 has not independently confirmed the accuracy of these methods. They are for reference only. |
||||
| 体内研究 (In Vivo) |
Ruxolitinib (INCB018424 sulfate; 180 mg/kg, orally, twice a day) results in survive rate of greater than 90% by day 22 and markedly reduces splenomegaly and circulating levels of inflammatory cytokines, and preferentially eliminated neoplastic cells, resulting in significantly prolonged survival without myelosuppressive or immunosuppressive effects in a JAK2V617F-driven mouse model[1]. 上海金畔生物科技有限公司 has not independently confirmed the accuracy of these methods. They are for reference only. |
||||
| Clinical Trial |
|
||||
| 分子量 |
404.44 |
||||
| Formula |
C17H20N6O4S |
||||
| CAS 号 |
1092939-16-6 |
||||
| 中文名称 |
鲁索利替尼硫酸盐 |
||||
| 运输条件 |
Room temperature in continental US; may vary elsewhere. |
||||
| 储存方式 |
Please store the product under the recommended conditions in the Certificate of Analysis. |
||||
| 参考文献 |
|
| Kinase Assay [1] |
Recombinant proteins are expressed using Sf21 cells and baculovirus vectors and purified with affinity chromatography. JAK kinase assays use a homogeneous time-resolved fluorescence assay with the peptide substrate (-EQEDEPEGDYFEWLE). Each enzyme reaction is carried out with Ruxolitinib or control, JAK enzyme, 500 nM peptide, adenosine triphosphate (ATP; 1mM), and 2% dimethyl sulfoxide (DMSO) for 1 hour. The 50% inhibitory concentration (IC50) is calculated as Ruxolitinib concentration required for inhibition of 50% of the fluorescent signal[1]. 上海金畔生物科技有限公司 has not independently confirmed the accuracy of these methods. They are for reference only. |
|---|---|
| Cell Assay [1] |
Cells are seeded at 2 × 103/well of white bottom 96-well plates, treated with Ruxolitinib from DMSO stocks (0.2% final DMSO concentration), and incubated for 48 hours at 37°C with 5% CO2. Viability is measured by cellular ATP determination using the Cell-Titer Glo luciferase reagent or viable cell counting. Values are transformed to percent inhibition relative to vehicle control, and IC50 curves are fitted according to nonlinear regression analysis of the data using PRISM GraphPad[1]. 上海金畔生物科技有限公司 has not independently confirmed the accuracy of these methods. They are for reference only. |
| Animal Administration [1] |
Mice[1] 上海金畔生物科技有限公司 has not independently confirmed the accuracy of these methods. They are for reference only. |
| 参考文献 |
|
所有产品仅用作科学研究或药证申报,我们不为任何个人用途提供产品和服务
