上海金畔生物科技有限公司为生命科学和医药研发人员提供生物活性分子抑制剂、激动剂、特异性抑制剂、化合物库、重组蛋白,专注于信号通路和疾病研究领域。
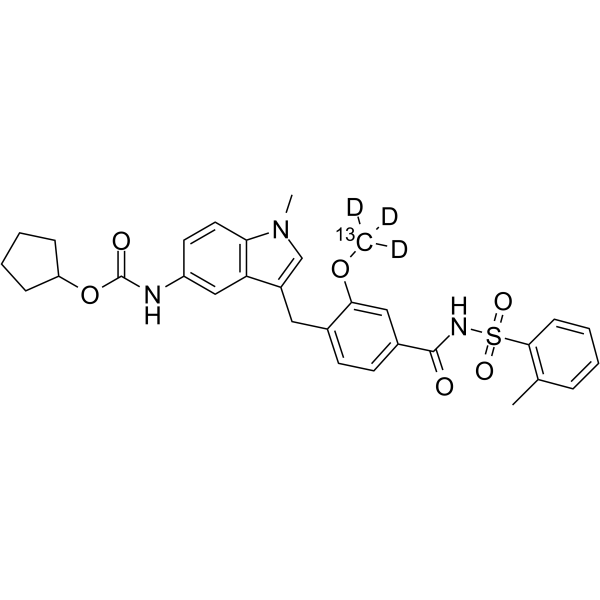
Zafirlukast-13C,d3 (Synonyms: ICI 204219-13C,d3)
Zafirlukast-13C,d3 是一种 13C- 和氘代标记的 Zafirlukast。Zafirlukast 是一种有效的具有口服活性的白三烯 D4 (LTD4) 受体拮抗剂。Zafirlukast 具有平喘、抗炎和抗菌作用。
Zafirlukast-13C,d3 Chemical Structure
| 规格 | 是否有货 | ||
|---|---|---|---|
| 100 mg | 询价 | ||
| 250 mg | 询价 | ||
| 500 mg | 询价 |
* Please select Quantity before adding items.
| 生物活性 |
Zafirlukast-13C,d3 is the 13C- and deuterium labeled. Zafirlukast (ICI 204219) is a potent orally active leukotriene D4 (LTD4) receptor antagonist. Zafirlukast shows anti-asthmatic, anti-inflammatory and anti-bacterial effects. |
|---|---|
| 体外研究 (In Vitro) |
Stable heavy isotopes of hydrogen, carbon, and other elements have been incorporated into drug molecules, largely as tracers for quantitation during the drug development process. Deuteration has gained attention because of its potential to affect the pharmacokinetic and metabolic profiles of drugs[44]. Shanghai Jinpan Biotech Co Ltd has not independently confirmed the accuracy of these methods. They are for reference only. |
| 分子量 |
579.69 |
| Formula |
C3013CH30D3N3O6S |
| 运输条件 |
Room temperature in continental US; may vary elsewhere. |
| 储存方式 |
Please store the product under the recommended conditions in the Certificate of Analysis. |
| 参考文献 |
|
所有产品仅用作科学研究或药证申报,我们不为任何个人用途提供产品和服务
