上海金畔生物科技有限公司为生命科学和医药研发人员提供生物活性分子抑制剂、激动剂、特异性抑制剂、化合物库、重组蛋白,专注于信号通路和疾病研究领域。
Doxorubicin (Synonyms: Hydroxydaunorubicin)
Doxorubicin (Hydroxydaunorubicin) 是一种有细胞毒性的蒽环类抗生素,常用作肿瘤化疗剂。Doxorubicin 抑制拓扑异构酶 II (topoisomerase-II),IC50 为 2.67 μM,从而抑制 DNA 复制。Doxorubicin 下调 AMPK 的基础磷酸化以及下游 acetyl-CoA 羧化酶。Doxorubicin 诱导凋亡 (apoptosis) 和自噬 (autophagy)。Doxorubicin 抑制人 DNA 拓扑异构酶 I (topoisomerase I),IC50 为 0.8 μM。
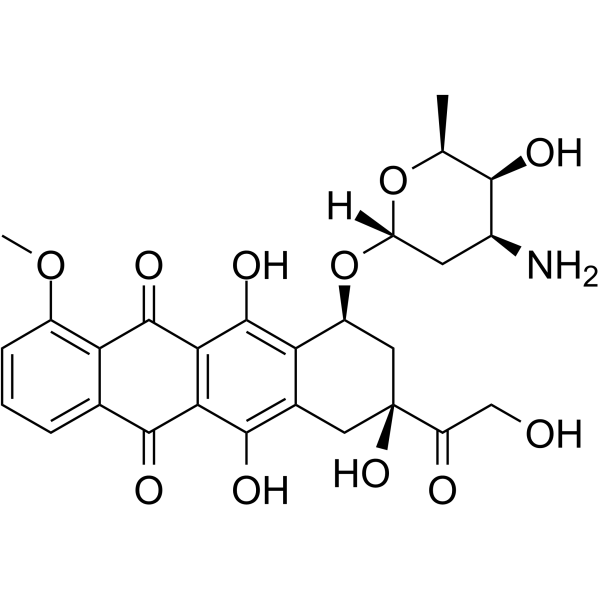
Doxorubicin Chemical Structure
CAS No. : 23214-92-8
| 规格 | 是否有货 | ||
|---|---|---|---|
| 5 mg | 询价 | ||
| 10 mg | 询价 | ||
| 25 mg | 询价 |
* Please select Quantity before adding items.
| 生物活性 |
Doxorubicin (Hydroxydaunorubicin), a cytotoxic anthracycline antibiotic, is an anti-cancer chemotherapy agent. Doxorubicin inhibits topoisomerase II with an IC50 of 2.67 μM, thus stopping DNA replication. Doxorubicin reduces basal phosphorylation of AMPK and its downstream target acetyl-CoA carboxylase. Doxorubicin induces apoptosis and autophagy[1][2]. Doxorubicin inhibits human DNA topoisomerase I with an IC50 of 0.8 μM[3]. |
||||
|---|---|---|---|---|---|
| IC50 & Target[1][2][3][7] |
|
||||
| 体外研究 (In Vitro) |
Combination of Doxorubicin (Hydroxydaunorubicin) and Simvastatin in the highest tested concentrations (2 μM and 10 μM, respec-tively) kills 97% of the Hela cells[4]. Shanghai Jinpan Biotech Co Ltd has not independently confirmed the accuracy of these methods. They are for reference only. |
||||
| 体内研究 (In Vivo) |
Mice bearing PC3 xenografts are injected with 2, 4 or 8 mg/kg Doxorubicin (Hydroxydaunorubicin) and tumor volume is measured over time. A dose of 2 mg/kg does not affect tumor growth while higher dosages delay tumor growth initially (p<0.05 at days 18 and 22), 4 mg/kg or 8 mg/kg Doxorubicin significantly reduces levels of c-FLIP in PC3 xenografts[5]. A single intraperitoneal injection 10 mg/kg (Doxorubicin 1) is administered in rats, 10 daily intraperitoneal injections of 1 mg/kg (Doxorubicin 2), or in 5 weekly intraperitoneal injections of 2 mg/kg (Doxorubicin 3). An 80% mortality rate is observed at day 28 in Doxorubicin 1, whereas Doxorubicin 2 and Doxorubicin 3 reached 80% mortality at days 107 and 98, respectively. Fractional shortening decreased by 30% at week 2 in Doxorubicin DOX1, 55% at week 13 in Doxorubicin 2, and 42% at week 13 in Doxorubicin 3[6]. Shanghai Jinpan Biotech Co Ltd has not independently confirmed the accuracy of these methods. They are for reference only. |
||||
| 分子量 |
543.52 |
||||
| Formula |
C27H29NO11 |
||||
| CAS 号 |
23214-92-8 |
||||
| 中文名称 |
阿霉素;多柔比星 |
||||
| 运输条件 |
Room temperature in continental US; may vary elsewhere. |
||||
| 储存方式 |
Please store the product under the recommended conditions in the Certificate of Analysis. |
||||
| 溶解性数据 |
In Vivo:
|
||||
| 参考文献 |
|
| Cell Assay [4] |
160 μL of Hela cells suspension (3×104 cell/mL) is dispensed into three 96-well U-bottom microplates and incubated for 24 h at 37°C in a fully humidified atmosphere of 5% CO2. In plate 1, serial dilutions of Doxorubicin (20 μL; final concentration, 0.1-2 μM) and Simvastatin (20 μL; final concentration, 0.25-2 μM) are added to a final volume of 200 μL and incubated for another 72 h. In plates 2 and 3 serial dilutions of each drug (Simvastatin or Doxorubicin, 40 μL) are added. After an incubation period of 24 h, the medium is aspirated and the cells are washed in PBS. Then, serial dilutions of other drug (40 μL) are added and supplemented with culture medium to a final volume of 200 μL, and incubated for 48 h. Doxorubicin and Simvastatin are used individually as positive controls (40 μL in each well), and the cells treated only with solvent are considered as negative controls. To evaluate cell survival, 20 μL of MTT solution (5 mg/mL in PBS) is added to each well and incubated for 3 h. Then the media is replaced with 150 μL of DMSO, and complete solubilization of formazan crystals is achieved by repeated pipetting of the solution. Absorbance is then determined at 540 nm by an ELISA plate reader. Each drug concentration is assayed in 4 or 8 wells and repeated 3 times. The cytotoxic/cytostatic effect of Doxorubicin is expressed as the relative viability (% control) and calculated. Percentage of cell survival in the negative control is assumed as 100. Relative viability=(experimental absorbance-background absorbance)/ (absorbance of untreated controls-background absorbance)×100 %[4]. Shanghai Jinpan Biotech Co Ltd has not independently confirmed the accuracy of these methods. They are for reference only. |
|---|---|
| Animal Administration [5][6] |
Mice[5] Shanghai Jinpan Biotech Co Ltd has not independently confirmed the accuracy of these methods. They are for reference only. |
| 参考文献 |
|
所有产品仅用作科学研究或药证申报,我们不为任何个人用途提供产品和服务
