上海金畔生物科技有限公司为生命科学和医药研发人员提供生物活性分子抑制剂、激动剂、特异性抑制剂、化合物库、重组蛋白,专注于信号通路和疾病研究领域。
CNDAC
CNDAC 是 sapacitabine 的有效代谢物,为一种核苷类似物。
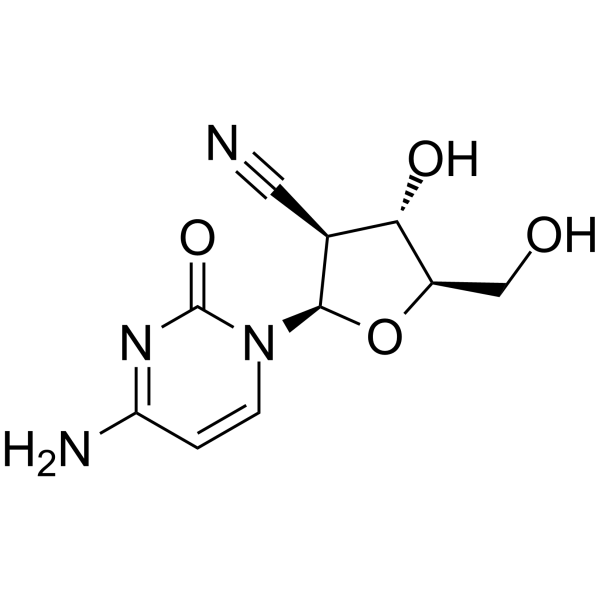
CNDAC Chemical Structure
CAS No. : 135598-68-4
| 规格 | 是否有货 | ||
|---|---|---|---|
| 100 mg | 询价 | ||
| 250 mg | 询价 | ||
| 500 mg | 询价 |
* Please select Quantity before adding items.
CNDAC 的其他形式现货产品:
| 生物活性 |
CNDAC is a major metabolite of oral drug sapacitabine, and a nucleoside analog. |
|---|---|
| 体外研究 (In Vitro) |
CNDAC-induced SSBs can be repaired by the transcription-coupled nucleotide excision repair pathway, whereas lethal DSBs are mainly repaired through homologous recombination. Deficiency in two Rad51 paralogs, Rad51D and XRCC3, greatly sensitize cells to CNDAC. The Rad51D-null cell line is approximately 50-fold more sensitive to CNDAC (IC50=0.006 µM) compared to 51D1.3, the Rad51D-repleted line (IC50=0.32 µM)[1]. CNDAC shows inhibitory activity against HL-60 and THP-1 cells with IC50s of 1.58 µM and 0.84 µM. CNDAC (10 μM) results in a significant drop in cell survival compared to the untreated on days 4, 7, and 14. CNDAC is more effective at reducing viability and inducing apoptosis than ara-C at equivalent concentrations in the THP-1 cell line, which is defined as displaying resistance to ara-C[2]. CNDAC induces DSBs, which are products of replication, rather than a consequence of induction of apoptosis. CNDAC causes DNA damage, and DNA-PK and ATR are dispensable for cell survival. CNDAC exhibits potent activity against human fibroblasts deficient in ATM or transfected with an empty vector, approximately 30-fold more than cells repleted with full-length ATM cDNA, with IC50s of 0.01 μM and 0.3 μM, respectively. CNDAC-induced DNA damage is repaired through the homologous recombination pathway[3]. Shanghai Jinpan Biotech Co Ltd has not independently confirmed the accuracy of these methods. They are for reference only. |
| 分子量 |
252.23 |
| Formula |
C10H12N4O4 |
| CAS 号 |
135598-68-4 |
| 运输条件 |
Room temperature in continental US; may vary elsewhere. |
| 储存方式 |
Please store the product under the recommended conditions in the Certificate of Analysis. |
| 参考文献 |
|
| Cell Assay [2] |
1×106 primary BM and PB cells are treated with 1 μM (low), 10 μM (medium), and 100 μM (high) of ara-C or CNDAC or 0.005 μM (low), 0.05 μM (medium) and 0.5 μM (high) mitoxantrone in 24 well plates at 37°C, 5% CO2, and 100% humidity for 4 days. Appropriate untreated controls are included. Postdrug treatment, both PB and BM non-adherent cells are washed to remove compound, replated on M2-10B4 stromal layers, and reincubated at 37°C, 5% CO2, 100% humidity. Cells are analyzed immediately posttreatment and following 3, 7, and 31 days postdrug removal. Shanghai Jinpan Biotech Co Ltd has not independently confirmed the accuracy of these methods. They are for reference only. |
|---|---|
| 参考文献 |
|
所有产品仅用作科学研究或药证申报,我们不为任何个人用途提供产品和服务